Lithium-ion batteries power the lives of millions of people each day. From laptops and cell phones to hybrids and electric cars, this technology is growing in popularity due to its light weight, high energy density, and ability to recharge.
So how does it work?
LIBs have an anode and electrode, as well as an electrolyte in three main components. When LIBs is charged, lithium ions are removed from the cathode electrode. The decomposition of lithium ions then travels through the electrolyte and transfers into the anode electrode, and the energy is stored in LIBs during this cycle. When the LIBs stop storing, the lithium ions move back to the cathode electrode; and the stored energy has been released.
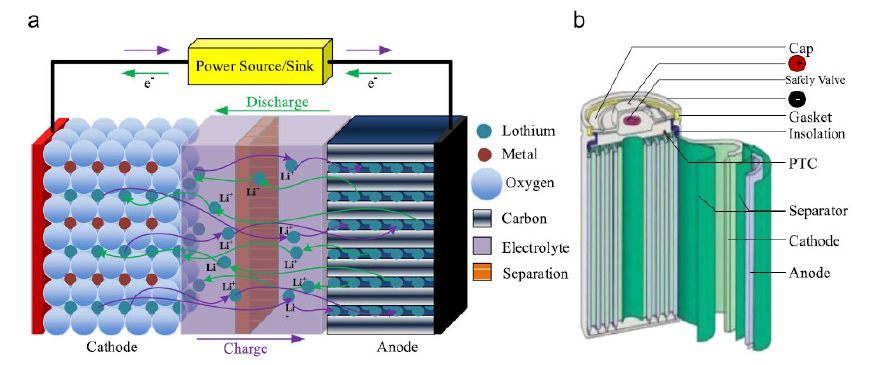
The two most common concepts associated with batteries are energy density and power density. Energy density is measured in watt-hours per kilogram (Wh/kg) and is the amount of energy the battery can store with respect to its mass. Power density is measured in watts per kilogram (W/kg) and is the amount of power that can be generated by the battery with respect to its mass.
The Vehicle Technologies Office works on increasing the energy density of batteries, while reducing the cost, and maintaining an acceptable power density.